Sodium citrate is a commonly used chemical component in the food industry as a food additive as a flavouring agent or as a preservative. E331 is the most common flavouring agent seen. Sodium citrate is the compound formed after the alkalinizing activity and is the sodium salt of citrate. Upon absorption, sodium citrate dissociates into sodium cations and citrate anions.
Properties Of Sodium Citrate
| Chemical formula | C6H5O7Na3 |
| Molecular weight | 258.068 g/mol (anhydrous)
294.10 g/mol (dihydrate) |
| Density | 1.7 g/cm3 |
| Chemical names | Sodium citrate tribasic dihydrate, Citric acid trisodium salt dihydrate and Trisodium citrate |
| Boiling point | Decomposes |
| Melting point | > 300 °C (572 °F; 573 K) |
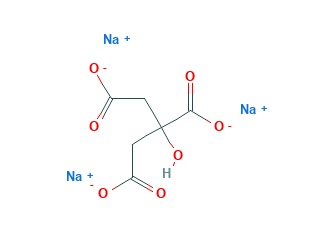
Sodium Citrate Structural Formula
Sodium citrate is an anticoagulant and hence used in milk-based foods like milk powder, yogurt, jams, sweets, ice cream and many more. The chemical structure of Sodium citrate is shown in the figure below.
For more information on any chemical compound, refer BYJU’S.
Comments